Introduction
In a broader sense ethical guidelines on biomedical research, in short, ‘Bioethics’, are the principles and standards which deal with the ethical issues arising from advances in the field of medicine and science. It relates to the relationship between life sciences, biotechnology, medicine and law. The term Bioethics is derived from two Greek words, ‘bios’ meaning life and ‘ethos’ meaning behaviour[1]. Biomedical ethics not just deal with humans but also encompasses plants and animals within its scope. A case in point is the banning of Bt Brinjal and HT Cotton by the Indian government because unethical issues involved[2].
The whole world united and raised their voice against the inhumane treatment of the humans, after the horrors of experiments conducted by the German scientists in World War II came into light. It led to formation of the Nuremberg code, 1947[3]. It was the first international code for ethics in clinical research laying down the guidelines for research on human beings. But the code was not being followed by some researchers as is evident from the historical cases like Tuskegee Syphilis experiment[4] and Human Radiation experiments[5]. This led to the World medical Association (WMA) developing a set of guidelines to safeguard the rights and dignity of the human participants in the clinical research. These guidelines were called the ‘Declaration of Helsinki’, 1964[6]. When the pharmaceutical industries of the developing and underdeveloped countries also started showing their keen interest in carrying out research experiments, the Council for international organisation of medical sciences (CIOMS) in association with the world health Organisation (WHO) came up with the International ethical guidelines for biomedical research involving human subjects in 1982[7].
Historical Background in Relation to India
In the year 1980, the Indian Council for Medical Research (ICMR) came up with a set of ethical guidelines which dealt with the clinical trials, informed consent, ethics committee and also how to conduct the research when it involves children, the mentally disadvantaged and those with diminished autonomy. With the passage of time modern technologies and new methods of conducting biological research seeped their way in and this led to the revision of the current ethical guidelines. So, the ICMR brought in the Pink Book; it was later on again revised in the year 2000 and was called the Blue Book. The ethical guidelines mentioned in the Blue Book indirectly got converted into law as they were incorporated in the amendments to the Indian Medical Council Act, 1956[8] and Drugs and Cosmetics Act, 1940 (D&C Act) through Schedule Y[9]. Also, every individual or organisation which is involved in conducting research is obligated to follow the Indian Good Clinical Practice guidelines[10] which are taken from the Good Clinical Practice (GCP) guidelines formulated by the International Conference on Harmonisation (ICH). But, because of rapid advancement in the field of biomedical research like Stem Cell research, Radio pharmaceuticals, Bioterrorism, Human genetics, Organ transplantation, including Fetal tissue transplantation, there is a constant need to revise the ethical guidelines. Therefore, just few years back the ICMR came up with the National Ethical Guidelines for Biomedical and Health Research involving Human Participants, 2018[11].
Overview of the ICMR’s National Ethical Guidelines for Biomedical and Health Research involving Human Participants, 2018
The ICMR ethical guidelines are categorised into 12 principles[12].
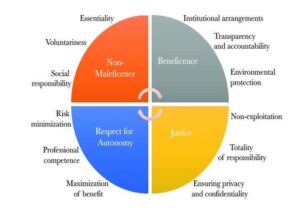
Principle of Essentiality:
Human participants are considered to be absolutely essential and research on them has to be conducted by the appropriate and responsible body of persons who are external to the particular research.
Principal of Voluntariness, Informed Consent and Community Agreement:
The research participants should be made aware of the nature of research and the probable outcomes of the experiment. Based on this knowledge the research participants should be asked to make an independent decision without any influence from the side of researchers. When there is involvement of any community or group of persons in the research, these principles of informed consent and voluntariness should apply to the whole community and also to each individual person who is the participant in the research or experiment.
Principle of Non-Exploitation:
The research participants should be paid for their involvement in the clinical research or experiment. Every research protocol should include provisions of compensation for human participants either through insurance cover or any other means to cover all the potential risk.
Principle of Privacy and Confidentiality:
All the data gathered from the clinical research or experiment should be kept confidential to preserve the identity of the human participants and should be disclosed only for any legal and/or scientific reasons.
Principle of Precaution and Risk Minimisation:
The research participants should be protected from all or any harm and adverse events at all the stages of the clinical research or experiment (right from its beginning as a research idea, formulation of the research design, conduct of research and any subsequent applications).
Principle of Professional Competence:
The clinical research or experiment should be carried out only by competent and qualified persons in their respective fields.
Principle of Accountability and Transparency:
The data collected during the clinical research or experiment should be stored and retained, subject to principles of privacy and confidentiality, for a duration of at least five years so that it may be, if needed, scrutinised by the legal or administrative authorities. Also, the researchers should conduct clinical research or experiment in fair, impartial and transparent manner.
Principle of Maximisation of Public Interest and Distributive Justice:
The result derived from any clinical research or experiment should be used for the betterment of all humans, especially the human participants in the clinical trials or research and/or the community to which they belong.
Principle of Institutional Arrangements:
All the institutional arrangements required to be made in respect of the clinical research or experiment and its subsequent use or application should be made in a transparent manner.
Principle of Public Domain:
The results of any clinical research or experiment conducted should be made public either through publications or any other means. Even before the start of any clinical research or experiment, their details must be made public via the Clinical Trial Registry systems that allow free online access.
Principle of Totality of Responsibility:
All the researchers and other people who are directly or indirectly involved in the clinical research or experiments should take professional and moral responsibility for the due observance of all the principles and guidelines laid down in relation to such research or experiment.
Principal of Compliance:
All those who are involved in the clinical research or experiment should comply with the guidelines pertaining to that specific area of clinical research or experiment.
Are These Ethical Guidelines being Complied With?
India is known as the pharmacy of the world and many countries look towards India for conducting the clinical trials, but when it comes to adhering to the ethical guidelines while conducting such clinical trials, unfortunately India is seen as the one not complying with the same. There have been several past instances where researchers have flouted the ethical guidelines, like in case of the controversial drug trials being conducted by the doctors of government medical College Madhya Pradesh[13]. Another example of the blatant disregard to human rights in general and ethical guidelines in particular, is the Human Papilloma Virus (HPV) vaccine trial on the tribal girls of Telangana[14] A PIL in this regard was also filed in the Supreme Court of India titled as Kalpana Mehta & Ors. v. Union of India & Ors[15]. As per the government data obtained by Swastha Adhikar Manch (SAM), between January 2005 to November 2017, 4,967 people died during the course of drug trials and research and some 20,000 odd people have suffered adverse reactions in such trials. The pharmaceutical companies have given compensation to the families of deceased persons only in 187 of such cases[16]. As per the founder of SAM, Dr Amulya Nidhi, “India’s regulators have been unable to keep up with this explosion of drug trials and testing. For instance, an ethics committee is supposed to oversee every trial but at one point, in Chandigarh, there were 257 trials going on, but only one ethics committee was overseeing them”[17].
SAM has also filed a PIL in the Supreme Court of India titled as Swasthya Adhikar Manch, Indore vs Union of India[18] in which they have raised the question of illegal clinical trials that were conducted on adults, children and mentally ill patients across the country.
Also, when it comes to the clinical trials, the financial aspect is a big motivation for the researchers and the pharmaceutical companies to overlook the ethical guidelines while conducting clinical research or experiment. According to a report by Frost and Sullivan[19], a market consultancy company, the annual revenue of the pharmaceutical companies who have outsourced the clinical trials to the researchers has grown from $485 million in 2011 to over $1 billion today.
Factors Contributing to Non-Compliance of the Ethical Guidelines
Conflict of interest:
The term ‘conflict of interest’ refers to situations in which financial or other personal considerations may compromise, or have the appearance of compromising the investigators’ professional judgement in conducting or reporting research. The bias such conflicts may conceivably impart not only affects collection, analysis and interpretation of data, but also the hiring of staff, procurement of materials, sharing of results, choice of protocol, and the use of statistical methods[20]. Often senior physicians or surgeons or advisers are members on the board of pharmaceutical companies that sponsor clinical trials. This adversely affects the conducting of such clinical trials. Further, the researchers might be having certain relationship with the company or the organisation sponsoring the trials, therefore, as a result the researchers may draw some pecuniary or any other kind of benefits from such companies or organisations. Also, the researchers may have invested in the companies that are sponsoring the trials, which also leads to conflict of interest either between the sponsor company or the researchers or between the human participants and the researchers.
Inadequate Training:
In the rural areas or tier 2 & tier 3 cities, availability of trained staff and resources such as space or beds is very low. The staff training is not that efficient and happens only for an initial period rather than for substantial duration. This ultimately results in researchers not complying with the ethical guidelines. Further, the medical schools do have a proper or holistic course on ethical guidelines which researchers need to follow while conducting clinical trials or research. Ultimately the graduates, which later on becomes researchers, do not possess any knowledge or experience when it comes to following the ethical guidelines.
Legal and Regulatory Framework:
At the end, the ethical guidelines are merely guidelines in nature and are not backed by any statutory law and the researchers are expected to follow them on their own. Therefore, lack of legislation means there is no or very less evidence of violation of such guidelines. Further, because of somewhat limited regulatory oversight, particularly in rural areas, the researchers, institute or journal, as the case maybe, even after violating such guidelines are able to walk away freely. The Drug Controller General of India (DCGI) designates the Ethics Committees (EC) as an important regulator of ethical research, but both these authorities (EC & DCGI) seem to work in isolation. There is no proper communication between the Ethics Committees functional in the country and the DCGI.
Informed Consent:
Socio-economic issues like language barriers, religious influences and literacy levels prevent human participants from accurately understanding the clinical trials or the research. This leads to non-compliance with one of the fundamental ethical guidelines-informed consent. In India, because of multitude of languages, the accuracy of consent forms and participant information sheets may be compromised because of inadequate translation. Furthermore, people living in a group have a strong sense of community and participate in collective decision making, especially in rural areas. In a study that was carried out in a village of Haryana, the majority of the participants interviewed could decide on the clinical trial participation only after discussing it with community members[21]. So, it is not their individual consent but a decision taken under the influence or pressure of peers. Further, there is an issue of who all are competent to give their consent. Apart from ensuring independent and informed consent, it is equally important to look whether the person giving the consent is even capable of giving it on his/her own?
A leading case on the issue of informed consent is Samira Kohli v. Dr. Prabha Manchanda & Another.[22] in which it was held that consent forms an essential part of any medical procedure and is a requisite before performing any procedure on an individual. If there is no real consent then any act performed will be deemed unlawful.
Participants’ Recruitment and Retention:
Recruitment and retention of the human participants in clinical trial is crucial for the progress of such trials. So, at times researchers tend to follow unethical practices in order to recruit and retain the human participants. All the requirements which researchers need to follow because of the statutory laws or ethical guidelines are just met only on paper. Further, there are huge financial resources involved during clinical trials. Therefore, retaining test subjects for the sponsor companies or organisations all the way becomes much more important which ultimately leads to non- compliance with the laws and ethical guidelines.
Publication Bias and Pressure to Publish:
In order to secure a research funding and advance in their careers, sometimes researchers may resort to plagiarism or duplicate publication. This clearly distorts the evidence-based clinical research, which in effect undermines the ethical guidelines as well. Such practice artificially enlarges the researcher’s scientific work, distorting apparent productivity and giving undue advantage to the unethical researchers.
Ethics Committees-Challenges and Issues:
One of the principal protections which is offered to an individual taking part in clinical research is the Ethics Committee review. The objective of an Ethics Committee review is to safeguard the dignity, rights, safety and well-being of the research participants. The institutions and hospitals primarily focus on the financial aspects and tend to ignore the Ethics Committees, which are still grappling with the basic issues like inadequate or no Standard Operating Procedures and non-compliance with the Schedule Y recommendations of D&C Act, 1940.
As per report of WHO[23] there are less than 40 ethics committees in our country, which were properly constituted and functioning. According to another survey[24] conducted to evaluate the competence of the Ethics Committees it was found that the committees were not having the knowledge of Schedule Y norms and Indian Good Clinical Practice guidelines. Also, they were unable to enlist the essential documents for reviewing the clinical trials.
Further, the Ethics Committees have to deal with basic issues such as lack of trained manpower, inadequate space allotted for their operations, heavy workload, lack of administrative support and in adequate remuneration offered to the members serving on the Ethics Committees boards. It is also seen that many Ethics Committees members are ambiguous about their roles and responsibilities during a review process. The ICMR guidelines are also not legislated, therefore Ethics Committees are reluctant to act against those who violate the prescribed guidelines.
Lastly, it is also possible that the members of Ethics Committee may be having any kind of relationship either with the researcher or with the sponsors or organisors of clinical research trials. There may be instances where the Ethics Committee members may be biased towards one sponsor because of any kind of relationship which leads to damage to other competitors’ studies or trials. There may even be cases where Ethics Committees function just as independent committees and the ethics review is mere a business for them.
The way Forward
We have formulated many ethical guidelines for clinical research, but the moot question remains- are researchers or companies or organisations or journals following them diligently?
Obviously there remain some concerns like the ethical guidelines are just recommendations and not backed up by any statutory law; the doctors are trained to be good clinicians but are never taught even the fundamentals of ethical clinical research. Also, the Indian ethical guidelines are heavily influenced from the western countries’ guidelines and are prepared keeping in mind the western culture, which can lead to unsatisfactory implementation of the ethical guidelines in case of India.
In order to imbibe a feeling of responsibility, accountability and empathy towards the vulnerable populations, medical students can be taught the above-mentioned qualities during their study phase, whether it be at school or college level. They should be taken to the field trips where Below Poverty Line (BPL) households and vulnerable population lives and show them how these vulnerable population are unable to secure their rights and it is their duty, legal and ethical, to protect the rights of such vulnerable people.
Further, the researchers or the Ethics Committee members may be holding shares of the sponsor companies and this may lead to violation of some ethical guidelines on their part. But on the other hand, holding a negligible number of shares of any sponsor company may not affect their independence and impartiality. So, researchers or investigators must disclose if they are holding any shares in the sponsor companies to the government authorities. Also, government should come out with a scheme where researchers or investigators or Ethics Committee members could hold shares of the sponsor companies of a limited value beyond which the researchers or the Ethics Committee members may not be allowed to be part of the clinical trials being conducted by such sponsor companies. Such a practice is prevalent in some developed countries like USA and UK[25].
Lastly, instead of relying totally on Ethics Committees the government authorities and regulators must independently verify the clinical trials data and ensure that there are stringent in-built quality checks and that the investigators conduct research fairly and independently.
[1] https://www.ijsr.net/archive/v4i2/SUB151485.pdf
[2] https://pib.gov.in/newsite/PrintRelease.aspx?relid=191120
[3] https://media.tghn.org/medialibrary/2011/04/BMJ_No_7070_Volume_313_The_Nuremberg_Code.pdf
[4] https://en.wikipedia.org/wiki/Tuskegee_Syphilis_Study
[5] https://ahf.nuclearmuseum.org/ahf/history/human-radiation- experiments/#:~:text=They%20came%20up%20with%20a,at%20least%20one%20with%20americium.
[6] https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/#:~:text=The%20World%20Medical%20Association%20(WMA,identifiable%20human%20material%20and%20data.
[7] https://cioms.ch/wp-content/uploads/2017/01/WEB-CIOMS-EthicalGuidelines.pdf
[8] https://www.nmc.org.in/wp-content/uploads/2017/10/Complete-Act-1.pdf
[9] https://cdsco.gov.in/opencms/export/sites/CDSCO_WEB/Pdf-documents/acts_rules/2016DrugsandCosmeticsAct1940Rules1945.pdf
[10] https://rgcb.res.in/documents/Good-Clinical-Practice-Guideline.pdf
[11] https://ethics.ncdirindia.org/asset/pdf/ICMR_National_Ethical_Guidelines.pdf
[12] https://indiabioscience.org/columns/indian-scenario/crossing-boundaries-ethical-guidelines-in-biomedical-research
[13] https://www.indiatoday.in/india/north/story/illegal-drug-trials-human-guinea-pigs-100934-2012-05-02
[14] https://economictimes.indiatimes.com/industry/healthcare/biotech/healthcare/controversial-vaccine-studies-why-is-bill-melinda-gates-foundation-under-fire-from-critics-in-india/articleshow/41280050.cms?from=mdr
[15] 2018,7 SCC (1)
[16] https://www.thenationalnews.com/world/asia/thousands-of-indians-die-in-unethical-clinical-trials-1.770992
[17] Ibid
[18] 2013 SCC Online SC 900
[19] https://store.frost.com/indian-contract-research-organization-cro-market.html
[20] https://www.yalecancercenter.org/research/resources/crs/research_ethics_and_conflicts_of_interest_9-28-05_-_sara_rockwell_phd_92972_284_31150_v1.pdf
[21] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1733858/pdf/v030p00318.pdf
[22] (2008) 2 SCC 1
[23] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3371548/#ref7
[24] Ibid
